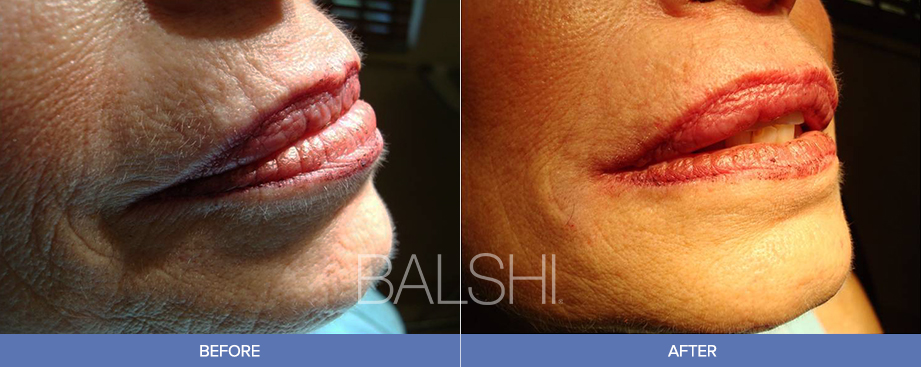
Indication: Restylane Silk® is indicated for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytids in patients over the age of 21. Restylane Silk® contains traces of gram-positive bacterial protein and is contraindicated for patients with allergies to such material or for patients with severe allergies that have required in-hospital treatment. Restylane Silk® should not be used by patients with bleeding disorders, with hypersensitivity to amide-type local anesthetics, such as lidocaine, under the age of 22, or by women who are pregnant or breastfeeding. Restylane Silk® should not be injected anywhere except the dermis or lip submucosa. Use of Restylane Silk® at the site of skin sores, pimples, rashes, hives, cysts, or infection should be postponed until healing is complete. The most commonly observed side effects are swelling, tenderness, bruising, pain, and redness at the injection site. These are typically mild in severity and resolve in 2-7 days after treatment. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis and scarring at the injection site. Do not implant into blood vessels. Use with caution in patients recently treated with anticoagulant or platelet inhibitors to avoid bleeding and bruising. Treatment volume should be limited to 1.5 mL per lip per treatment and 1.0 mL for perioral rhytid correction, as greater amounts significantly increase moderate and severe injection site reactions. The safety or effectiveness of treatment in areas other than lips and perioral rhytids has not been established in controlled clinical studies.